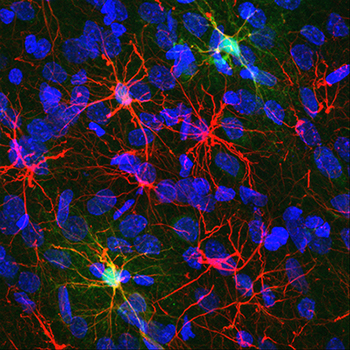
 Research in the Reissner lab is designed to understand how experience, particularly drug abuse, affects the structural and functional interactions between neurons and astrocytes. Astrocytes are star-like cells whose name is derived from the Greek “astron”, meaning star. Astrocytes are the most abundant cell type of the brain, and they participate in critical, bidirectional communication with neurons. Images of astrocytes from the nucleus accumbens of a rat brain are shown in figure 1, on the left. The marker protein glial fibrillary acidic protein (GFAP) is shown in red. Cell bodies of all cells, regardless of type, are marked in blue. The two brightest astrocytes have been engineered to express a protein (GCaMP) which fluoresces brightly upon calcium binding.
Research in the Reissner lab is designed to understand how experience, particularly drug abuse, affects the structural and functional interactions between neurons and astrocytes. Astrocytes are star-like cells whose name is derived from the Greek “astron”, meaning star. Astrocytes are the most abundant cell type of the brain, and they participate in critical, bidirectional communication with neurons. Images of astrocytes from the nucleus accumbens of a rat brain are shown in figure 1, on the left. The marker protein glial fibrillary acidic protein (GFAP) is shown in red. Cell bodies of all cells, regardless of type, are marked in blue. The two brightest astrocytes have been engineered to express a protein (GCaMP) which fluoresces brightly upon calcium binding.
Several lines of investigation in the Reissner lab indicate that cocaine abuse exerts changes in astrocytes within the brain’s reward circuitry, and that these changes may contribute to the cellular mechanisms which drive relapse to drug use following abstinence. For example, the Reissner lab recently showed that astrocytes are smaller and colocalize less with neurons following withdrawal from cocaine self-administration within the nucleus accumbens core, a brain region critical for the execution of motivated behaviors [1]. To do this, astrocytes, including the finest processes that act as fingers to reach out and touch synapses, are engineered to express a green fluorescent protein (Lck-GFP, different from the GCaMP in figure 1). High-resolution microscopy then allows us to create three-dimensional images of an entire, individual astrocyte, from which specialized software calculates measurements such as surface area and volume (figure 2, on the right). 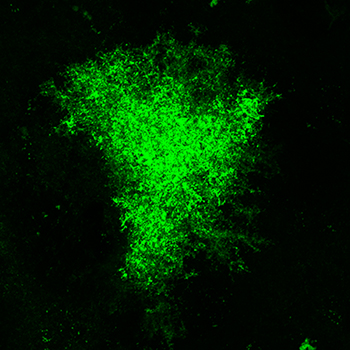 Note how much more complex the astrocyte in figure 2 is compared to figure 1! These are the same types of cells, but imaged in very different ways. Please see the movie below to see the comparison of a single astrocyte imaged with Lck-GFP against a background of cells in the same field imaged for expression of GFAP. Again, cell bodies of all cells are visible in blue (DAPI). The space-filling model shown in red is used for measurement of surface area and volume.
Note how much more complex the astrocyte in figure 2 is compared to figure 1! These are the same types of cells, but imaged in very different ways. Please see the movie below to see the comparison of a single astrocyte imaged with Lck-GFP against a background of cells in the same field imaged for expression of GFAP. Again, cell bodies of all cells are visible in blue (DAPI). The space-filling model shown in red is used for measurement of surface area and volume.
Identification of drug-induced adaptations in astrocytes can help to identify pharmacological treatments to assist against relapse to use of cocaine and other drugs. For example, besides reducing the size and synaptic colocalization of astrocyte processes, cocaine also leads to downregulation of GLT-1, an important protein localized to astrocyte membranes. Upregulation of GLT-1 has been shown to contribute to the ability of compounds such as N-acetylcysteine propentofylline, and ceftriaxone to reduce drug-seeking behaviors in rats, suggesting therapeutic potential for substance use disorders [2,3,4]. The Reissner lab recently extended this work to test whether the FDA approved medication, Riluzole, can also reduce cocaine seeking in rats [5]. Riluzole has many effects on neurons and astrocytes, including upregulation of GLT-1. This study found that in addition to reducing cocaine-seeking behavior in rats, administration of riluzole also increased GLT-1 expression, and normalized cocaine-induced changes in neuronal excitability in the prefrontal cortex.
In summary, members of the Reissner lab seek to understand the biology of neuron-astrocyte communication. Dr. Reissner is particularly interesting in understanding the long-lasting changes in neuron-astrocyte communicationwhich are induced by drugs of abuse, and whether these represent promising avenues for therapy against substance use disorders.
 Dr. Kathryn Reissner is an Associate Professor in the Behavioral and Integrative Neuroscience Program within the Department of Psychology and Neuroscience at UNC Chapel Hill. Her work examines how chronic self-administration of cocaine leads to modifications in cellular physiology and neuronastrocyte communication and how these modifications may contribute to long-term drug abuse. Learn more about her research online.
Dr. Kathryn Reissner is an Associate Professor in the Behavioral and Integrative Neuroscience Program within the Department of Psychology and Neuroscience at UNC Chapel Hill. Her work examines how chronic self-administration of cocaine leads to modifications in cellular physiology and neuronastrocyte communication and how these modifications may contribute to long-term drug abuse. Learn more about her research online.
[1]. M.D. Scofield, H. Li, B. Siemsen, K.L. Healey, P.K. Tran, N. Woronoff, H.A. Boger, P.W. Kalivas, K.J. Reissner (2016) Cocaine self-administration and extinction leads to reduced GFAP expression and morphometric features of astrocytes in the nucleus accumbens core. Biological Psychiatry, 80(3): 207-215.
[2]. K.J. Reissner, R.M. Brown, S. Spencer, P.K. Tran, C.A. Thomas, P.W. Kalivas. (2014) Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1 dependent mechanism. Neuropsychopharmacology 39(2):499-506.
[3]. K.J. Reissner, C.D. Gipson, P.K. Tran, L.A. Knackstedt, M.D. Scofield, P.W. Kalivas (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction Biology, 20(2):316-23.
[4]. A.L. LaCrosse, S.M. O’Donovan, M.T. Sepulveda-Orengo, R.E. McCullumsmith, K.J. Reissner, M. Schwendt, L.A. Knackstedt (2017) Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. Journal of Neuroscience, 37(24): 5809-5821.
[5]. K.J. Reissner, C.D. Gipson, P.K. Tran, L.A. Knackstedt, M.D. Scofield, P.W. Kalivas (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addiction Biology, 20(2):316-23.
M.T. Sepulveda-Orengo, J. Rojas, K.L. Healey, A. Auriemma, N. Woronoff, K.J. Reissner. Riluzole Impairs Cocaine Reinstatement and Restores Intrinsic Excitability in the Prefrontal Cortex. Neuropsychopharmacology, In press.

